Mécanismes moléculaires impliqués dans l'activité myogénique de la triiodothyronine (T3)
Date
1996Auteur
Marchal, S
Cassar-Malek, I
Rodier, A
Wrutniak, C
Cabello, G
Voir/
Metadata
Afficher la notice complèteRésumé
Depuis les travaux initiaux de Scow en 1 95 1 , de nombreuses
données obtenues in vivo ont établi l'influence des
hormones thyroïdiennes sur le développement musculaire.
Les travaux réalisés au cours des trois dernières années
grâce à la caractérisation des récepteurs nucléaires de la T3
ont permis d'élucider quelques mécanismes moléculaires
impliqués dans la stimulation de la différenciation musculaire
par cette hormone. Parallèlement à une activation de
la synthèse des facteurs myogéniques MyoD et Myogénine
observée dans les myoblastes murins (mais pas dans les
myoblastes aviaires) , l'inhibition de l'activité AP-l semble
constituer un mécanisme majeur de l'influence myogénique
de la T3. Cette voie d'action implique, à un moment précis
de la progression des myoblastes dans la voie de la différenciation
terminale, l'induction de la synthèse de RXR et
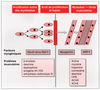
une stimulation transitoire de la voie de l'AMPc. The importance of thyroid hormone in the regulation of in vivo myogenic processes is well documented. This hormone stimulates muscle growth by increasing the fibers number and diameter. In addition, T3 affects the expression of different myosin isoforms, by a direct transcriptional pathway in cardiac muscle, indirectly in skeletal muscle. Last, according to the strong effect of this hormone upon mitochondriogenesis and mitochondrial activity, it could be also involved in the processes of metabolic differentiation. However, the molecular basis of these influences remained largely unknown, Cell culture experiments provide strong evidence that T3 stimulates myoblast differentiation, by increasing the rate of myoblast withdrawal from the cell cycle. Recent data suggest that in murine myoblasts, MyoD and myogenin genes transcription is directly stimulated by T3; however, as a similar phenomenon does not occur in avian myoblasts in which T3 displays a stronger myogenic influence, such a mechanism is probably not essential. Studies performed in avian cells (secondary cultures and QM7 line) indicate that inhibition of AP-1 activity is probably a crucial target involved in the T3 myogenic influence. Interestingly, this pathway is only effective at a specific stage of myoblast progression in the differentiation program: (1) the liganded c-erbAalpha1 T3 nuclear receptor represses AP-1 transcriptional activity only when RXR is expressed, an event induced by reducing the serum concentration in the culture medium to induce terminal differentiation; (2) at this stage, a T3-regulated transient increase in cAMP production stimulates CREB expression, and consequently its ability to repress AP-1 activity. As AP-1 activity represses BTG1 (an antiproliferative protein) expression, this event consequently allows BTGI to be synthetized. Moreover, T3 stimulates the nuclear import of this protein in parallel to an increase of myoblast exit from the cell cycle. Lastly, BTG1 overexpression, as a T3 treatment, stimulates myoblast terminal differentiation. All these data clearly suggest that AP-1 activity is a crucial target of T3, able to repress the expression of BTGI, a protein displaying a similar myogenic influence than T3. Moreover, as T3 and its receptor cannot inhibit AP-1 activity by themselves, this influence is only induced at a particular stage, thus providing protection against anticipated differentiation.
Pour citer ce document
Marchal, S ; Cassar-Malek, I ; Rodier, A ; Wrutniak, C ; Cabello, G, Mécanismes moléculaires impliqués dans l'activité myogénique de la triiodothyronine (T3), Med Sci (Paris), 1996, Vol. 12, N° 10; p.1065-76



