| |
| Med Sci (Paris). 34: 87–93. doi: 10.1051/medsci/201834f115.NPS - 2143 (hydrochloride) inhibits melanoma cancer cell proliferation and induces autophagy and apoptosis Shumei Wang,1 Liyun Qiu,2 Haiyan Song,3 and Ningning Dang3* 1Department of Community Medicine, Jinan Central Hospital affiliated to Shandong University, No.105 Jiefang Road, Jinan250013, Shandong Province, China 2Department of Pharmacy, Jinan Central Hospital affiliated to Shandong University, No.105 Jiefang Road, Jinan250013, Shandong Province, China 3Department of Dermatology, Jinan Central Hospital affiliated to Shandong University, No.105 Jiefang Road, Jinan250013, Shandong Province, China |
Melanoma is a common and aggressive skin cancer caused by the oncogenic transformation of melanocytes. NPS-2143 (hydrochloride) is a calcification drug that acts as an antagonist of the calcium-sensing receptor (CaSR) and consequently stimulates the release of parathyroid hormone. In the present work, we treated cells from the human melanoma cell line M14 to investigate the effects of NPS-2143 on melanoma cells and elucidate their underlying mechanisms. We observed that NPS-2143 inhibits the survival and proliferation of M14 cells and suppresses the migration and proliferation of M14 cells by inducing apoptosis. The Bax/Bcl‑2 ratio in M14 cells was enhanced by the NPS-2143 treatment, suggesting that the mitochondrial apoptotic pathway was activated. The expression and phosphorylation of proteins involved in the PI3K signaling pathway were altered by NPS-2143 treatment. Our data show that NPS-2143 impacts the viability and induces the apoptosis of melanoma M14 cells through its impact on the PI3K signaling pathway. It suggests that NPS-2143 could represent a promising candidate for melanoma treatment. Keywords: NPS-2143, melanoma cancer, M14, apoptosis, PI3K pathway |
Melanoma is a common skin cancer caused by hyper-proliferation of abnormal melanocytes. It typically occurs in the skin, but, although rarely, also in the mouth, intestines, brain or eyes [1- 3]. It is extremely aggressivet and accounts for a large proportion of death among skin tumors. They are most commonly found on the legs in women, and on the back in men [4]. The incidence of melanoma varies with ethnic group and geography [5]. Caucasians, for example, have a much higher incidence of melanoma than other ethnic groups. The prevalence of Caucasians living in Queensland, Australia, is up to 17/10 millions [6, 7]. Australia and New Zealand have the highest incidence of melanoma in the world [4]. Northern Europe and North America also have high rates of the disease, while in Asia, Africa and Latin America, it is relatively rare [4]. The primary cause of melanoma is ultraviolet light (UV) exposure, possibly from the sun or other sources, such as tanning devices [4, 8]. Exposure to sunlight is a risk factor, and the same risk factors include family history, presence of speckles, larger congenital melanocytic nevus and immature nevus syndrome [9]. Using sunscreen and avoiding UV light may prevent melanoma [4].Those with lots of moles, a family history, and people with poor immune function are at greater risk [10]. About 25% of melanomas develop from moles, including an increase in size, irregular margins, color changes, itching or skin rupture [4, 10]. Some rare genetic defects, such as xeroderma pigmentosum also increase risk [11]. The diagnosis is a biopsy of any concerning skin lesion [10]. Treatment is typically performed by surgical removal [10]. For most people, melanomas are cured by surgery if there is no spreading of the primary tumor; for patients with melanoma spreading, the survival rate can be improved by immunotherapy, biologic therapy, radiation therapy, or chemotherapy [10, 12]. The likelihood of its recurrence or metastasis depends on the thickness of the primary tumor, the rate of cell division, and whether the covered skin has ruptured [4]. When treated, the five-year survival rate in the United States is 98% among the patients with localized disease and 17% in the metastatic group [13]. NPS-2143 (hydrochloride) is a calcification drug that acts as an antagonist at the Calcium-Sensing Receptor (CaSR), and consequently stimulates the release of parathyroid hormone [14]. Calcified drugs have been studied for the potential treatments for osteoporosis. The first such compound, NPS-2143 is still widely used to study of CaSR receptor, as well as to design new calcification agents [15- 17]. NPS-2143 is the first recognized Ca2+ receptor antagonist (calcifying agent). NPS-2143 stimulates the secretion of parathyroid hormone (PTH) from bovine parathyroid cells, resulting in a rapid and large increase in plasma levels of PTH [14]. The pharmacodynamic properties of NPS-2143 together with the recently confirmed effects of this compound on bone formation support the view that the activity of oral calcium compounds for osteoporosis might provide a novel anabolic therapy [14]. Meanwhile, oral administration of NPS-2143 and subcutaneous infusion of 17beta-estradiol also increase bone turnover [18]. These SAR studies eventually led to the identification of NPS-2143 as a potent and orally active CaSR antagonist [15]. In this study, we aimed to investigate the effects of NPS-2143 on human melanoma and elucidate the underlying mechanisms. Herein, we reported that the in vitro treatment of melanoma cells with NPS-2143 inhibits the viability and induces the apoptosis of M14 melanoma cells through the PI3K signaling pathway. Our data suggest that NPS-2143 deserves further in vitro and preclinical in vivo studies to investigate whether it represents a new agent for melanoma cancer therapy. |
Reagents RPMI-1640 medium was purchased from HyClone Company (Logan, Utah. USA). Fetal goat serum was purchased from Gibco (USA). Antibiotics were from Sigma (USA) and DMSO was ordered from AMRESCO (USA). CCK-8 and 0.25% trypsin with EDTA were purchased from Beijing Solarbio science & technology company (Beijing, China). Annexin V-FITC/PI apoptosis kit was ordered from 4A Biotech Company (Beijing, China). The primary antibodies used were rabbit or mouse anti-ß-catenin (1:1000, PTG); -AKT CST (1:1000, CST); -p-AKT (1:1000, CST); -mTOR (1:1000, CST); -p-mTOR (1:1000, CST); -Bcl-2 (1:1000, PTG); Bax (1:1000, PTG); active-caspase3 (1:1000, PTG); p-70 (1:1000, PTG); GAPDH (1:5000, PTG), Cyclin D1 (1:1000, PTG); CDK4 (1:1000, PTG); CDK6 (1:1000, PTG); E-cad (1:1000, PTG); Notch1 (1:1000, Abcam); Hey1 (1:1000, PTG). HRP-conjugated sheep anti rabbit/mouse secondary antibodies (1:5000) were ordered from the PTG Company. Ultrapure RNA extraction kit, HiFiScript cDNA Synthesis Kit, fluorescence quantitative PCR kit UltraSYBR Mixture, RIPA Lysis Buffer, BCA Protein Assay Kit, Protease Inhibitor Cocktail were all purchased from Beijing Kangwei Century company (CwBio, Beijing, China). Matri-gel was ordered from BD company (USA). Transwell cell culture plates were ordered from Millipore company (USA). The LDS sample buffer was ordered from Invitrogen (USA). Protein Markers were ordered from ThermoScientific (USA). The ECL developer was ordered from PTG (USA). Cell culture and dose-dependent experiments The melanoma cancer cell line M14 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium containing 10% FBS, 100U/mL Penicillin and 0.1mg/mL streptomycin (culture medium) at 37°C in a 5% CO 2 humid atmosphere. Before each experiment, cells were trypsinized once at the logarithmic phase of growth. Dilutions of NPS-2143 were added to wells or dishes seeded with M14 cells the day before. Western blot Cells were cultured in a 6-well plate until 95% confluency. After culture in presence of NPS-2143 for 24h, cells were lysed with ice-cold RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0 and Protease inhibitors) and the protein concentration was determined by the BCA method. Protein samples were denatured by heating at 95°C for 5min and about 20 μg were loaded into each lane on a 10% SDS-PAGE gel. After transferring protein to a PVDF membrane, the membrane was blocked with 5% non-fat milk for 1h at room temperature and incubated with primary antibodies in blocking solution at 4°C overnight. The membrane was then washed with TBST 3 times, 5 min each, and incubated with secondary antibodies in blocking buffer at room temperature for 1h. After washing, the membrane was treated with the ECL substrate. Images were acquired by using darkroom development techniques for chemiluminescence. Grayscale values of band intensity were obtained by scanning with the QUANTITY ONE software. The value of the band intensity of GAPDH was used as the internal reference control. The relative expression of each protein was calculated as the protein intensity/internal GAPDH reference intensity. Cell Viability and Proliferation Assays M14 cells were trypsinized and counted and 1,000 cells in culture medium were added to each well of a 96-well plate. Different doses of NPS-2143 were then added and cells were cultured in a 5% CO 2 humid atmosphere at 37°C. Cell viability was evaluated every 24 hours by adding 10μL of CCK8 reagent. After incubation at 37°C for 90min, the Optical Density (O.D.) values were assessed at 450 nm. Clone formation efficiency assays Cells were cultured to 95% confluency and incubated with NPS-2143 for 24h. Cells were then trypsinized and counted. 500 cells were then added to each 10 cm dish containing 5 mL medium and the dishes were incubated at 37°C in a 5% CO 2 humid atmosphere and incubated for 1-2 weeks. One mL of culture medium containing the relevant concentration of NPS-2143 was added to the cultures every 3 days (and of PBS for control group). Cells were cultured until sufficiently large clones have formed. Cells were then harvested, washed carefully twice with PBS, and fixed with 5 mL 4% paraformaldehyde for 30 min followed by staining with 0.1% crystal violet for 30 min. After removing the crystal violet solution carefully and rinsing with tap water, the dishes with colonies were allowed to dry on the bench at room temperature. The numbers of colonies were counted and compared as well as the colony size with those obtained with the control group. Transwell invasion and migration assays Matrigel Matrix aliquot was thawed on ice and then stored at 4°C overnight. It was then diluted with serum-free 1640 medium at 1:6 and 100 µL were applied to each permeable support well of a 24-well plate and incubated at 37°C for 4h with coating buffer. The remaining coating buffer was carefully removed from the permeable support membrane without disturbing the layer of Matrigel on the membrane. One hundred μL and 600µL serum-free RPMI 1640 medium were then added to the inside and outside of the support well, respectively, and incubated at 37°C for 30 min. The coating buffer (containing 0.01M Tris pH 8.0, 0.7% NaCl and filtered with a 0.2 µM sterile filter unit) should be prepared before experiment and all pipette tips and pipettes kept in the cold before use. After incubation with NPS-2143 for 24h, cells were trypsinized and re-suspended in serum-free culture medium. One hundred µL cell suspension containing 1×105 cells were added to each 24-well invasion chambers and 500 µl culture medium containing 10% FBS were added to the outside part of the chamber. After incubation at 37°C overnight, non-invading cells were removed by using a cotton swab. Both sides of the chambers were then washed with PBS twice. Cells were fixed with 4% paraformaldehyde at room temperature for 30 min and stained with 0.1% crystal violet for 20 min. After washing with PBS, the filters were cut off and mounted on slides. The invading cells were then imaged and counted under the microscope. The design of the migration experiment procedure was similar to that of the invasion experiment. Cell apoptosis analysis with flow cytometer After incubation with NPS-2143 for 24h in culture medium, cells were treated with serum-free medium for a further 24h. Cells were then trypsinized with EDTA-free trypsin. After washing with ice-cold PBS, cells were re-suspended in 10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl 2 buffer. Cell concentration was then adjusted to 1-5×10 6 cells/mL. Five μL of AnnexinV-FITC were added to 100 μL cell suspension, incubated for 20 min at room temperature in dark. Ten μL of 20 μg/mL PI were then added and incubated for 2 min. Labeled cells were analyzed with a flow cytometer (BD FACS Canto II, BD Biosciences, San Jose, CA, USA). Viable cells were negative for both PI and Annexin V, while early apoptotic cells were positive for Annexin V and negative for PI. Late apoptotic dead cells showed both Annexin V and PI positivity. The apoptotic rate was calculated using the Flowjo software. Statistical analysis Statistical analysis was performed by using the SPSS 18.0 software. Results were expressed as means ± standard deviation (S.D.), as indicated in the figure legends. Data were representative of three independent experiments performed in triplicate. The student t-test was used to determine the significance for all pairwise comparisons of interest. Differences were considered statistically significant for values of P<0.05. |
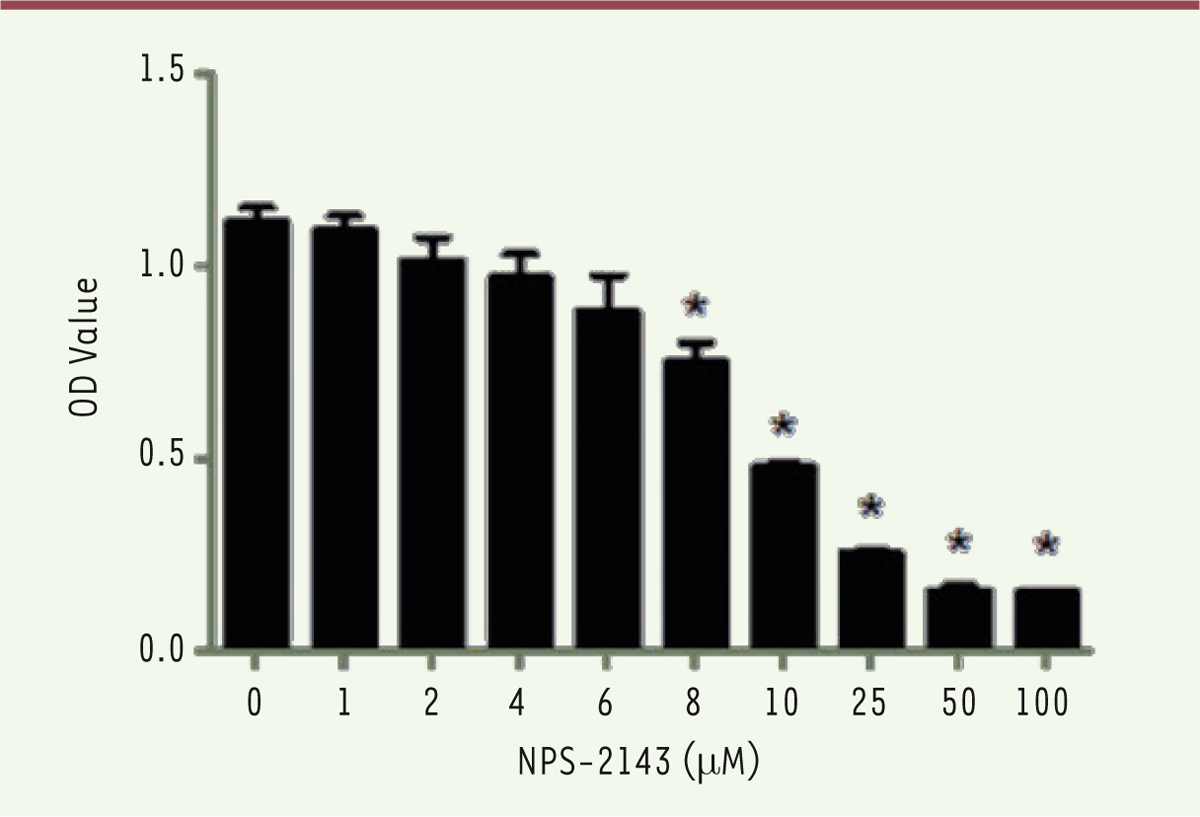
Dose-dependent effect of NPS-2143 on the viability of melanoma M14 cancer cells To evaluate the optimal concentration of NPS-2143 for M14 treatment, dilutions of the drug were incubated with M14 cells in a 96-well plate. After 24h of culture, a CCK8 assay was performed and the 450nm O.D. values were determined. There was no effect on M14 cells when concentrations of NPS-2143 below 6 µM were used. However, M14 cells showed a significant decrease in the O.D. value at 8 µM. The O.D. values even decreased to 0.47 at 10 µM. When the NPS-2143 dose was increased to 25 µM, the O.D. value decreased down to 0.25, with no more significant change when the NPS-2143 concentrations were increased up to 50 µM. This result suggests that NPS-2143 has a dose-dependent inhibitory effect on M14 cells ( Figure 1). In this experiment, the IC50 was 9.509µM, a dose that was therefore used as the treating concentration in the following experiments.
 | Figure 1.
NPS-2143 (hydrochloride) inhibits viability of M14 cells in a dose-dependent manner. M14 cells were treated with different concentrations of NPS-2143 (0, 1, 2, 4, 6, 8, 10, 25, 50, and 100µM) for 24h, then O.D. values of NPS-2143-treated M14 cells was detected at 450nm. IC50 = 9.509 µM. |
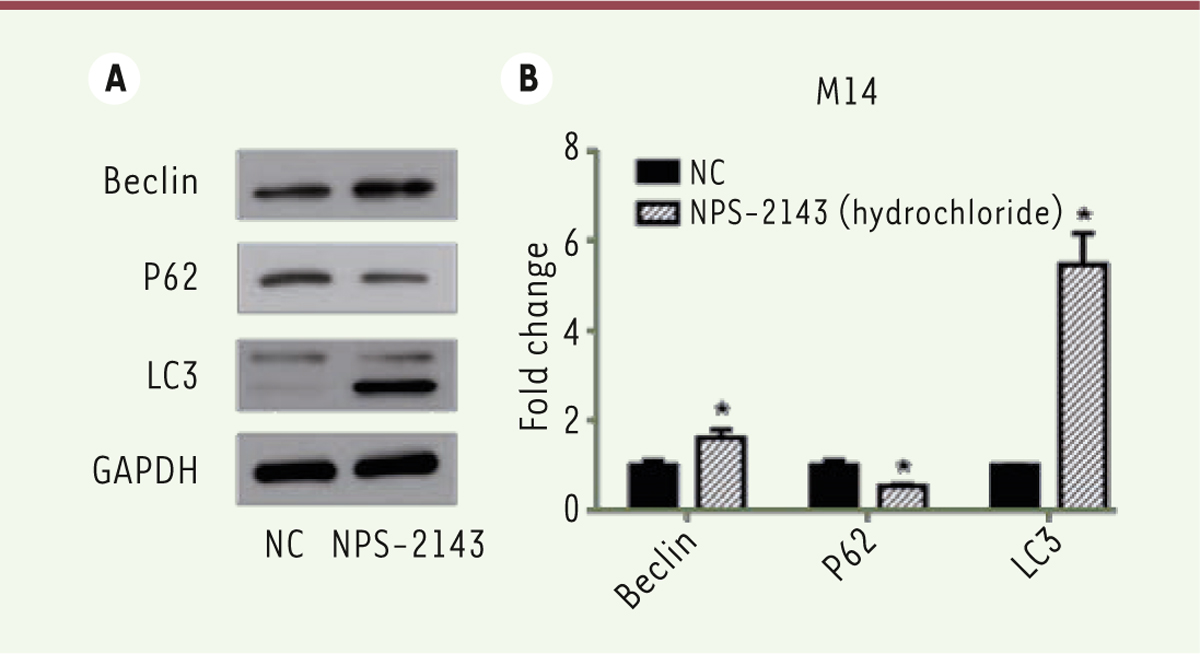
NPS-2143 (hydrochloride) treatment can promote the M14 cell autophagy We also assessed the expression of various proteins related to the autophagy process. Twenty-four hours after treatment with NPS-2143, cells were harvested and lysed. Western-blot analyses showed that the expression of Beclin and LC3 were significantly increased after treatment with NPS-2143 while that of P62 decreased ( Figure 2A and B). These results suggest that NPS-2143 (hydrochloride) can promote autophagy of M14 melanoma cells.
 | Figure 2.
NPS-2143 (hydrochloride) promotes M14 cell autophagy. A. Western blots r show that the expression of Beclin and LC3 is raised following the treatment of M14 cells by NPS-2143, while that of P62 decreases. B. The values of the band intensity represent the densitometric estimation of each band normalized to GAPDH (*P<0.05). |
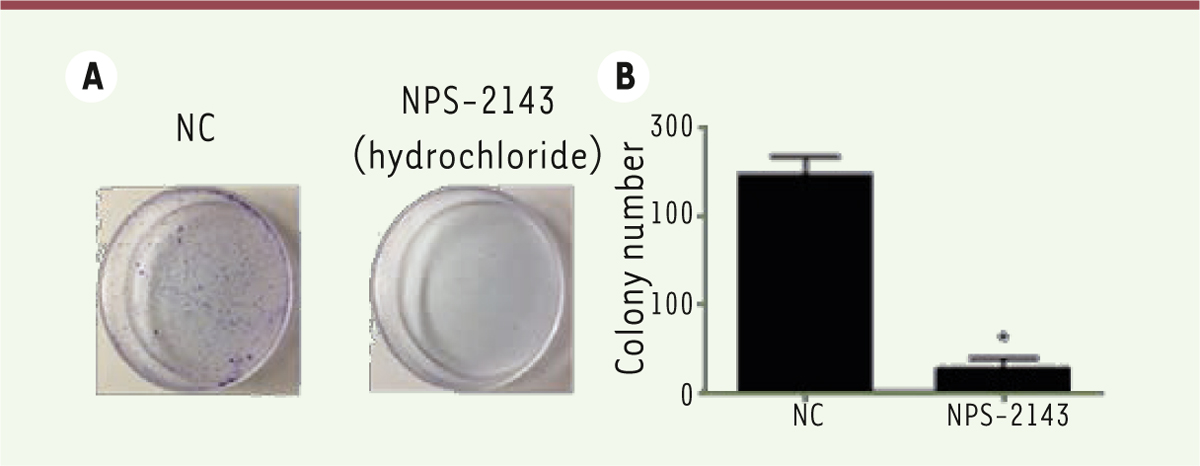
NPS-2143 significantly decreases the proliferation of M14 cells We then evaluated the effect of NPS-2143 on the proliferation of M14 melanoma cancer cells. As shown by a CCK8 assay, 24h after NPS-2143 treatment, the proliferation ability of M14 cells was significantly decreased ( Figure 3 A and B).
 | Figure 3.
NPS-2143 significantly decreases proliferation of M14 cells. Twenty-four hours after treatment, the proliferation of M14 cells was significantly decreased (*
P < 0.05). CCK8 assays and colony formation assay were performed to evaluate whether the treatment of M14 cells by NPS-2143 affects cell proliferation. A. Colony formation assay showed a significant decrease in the proliferation rate of M14 cells as compared to the NC control. B. Histograms of colony numbers (*
P < 0.05). |
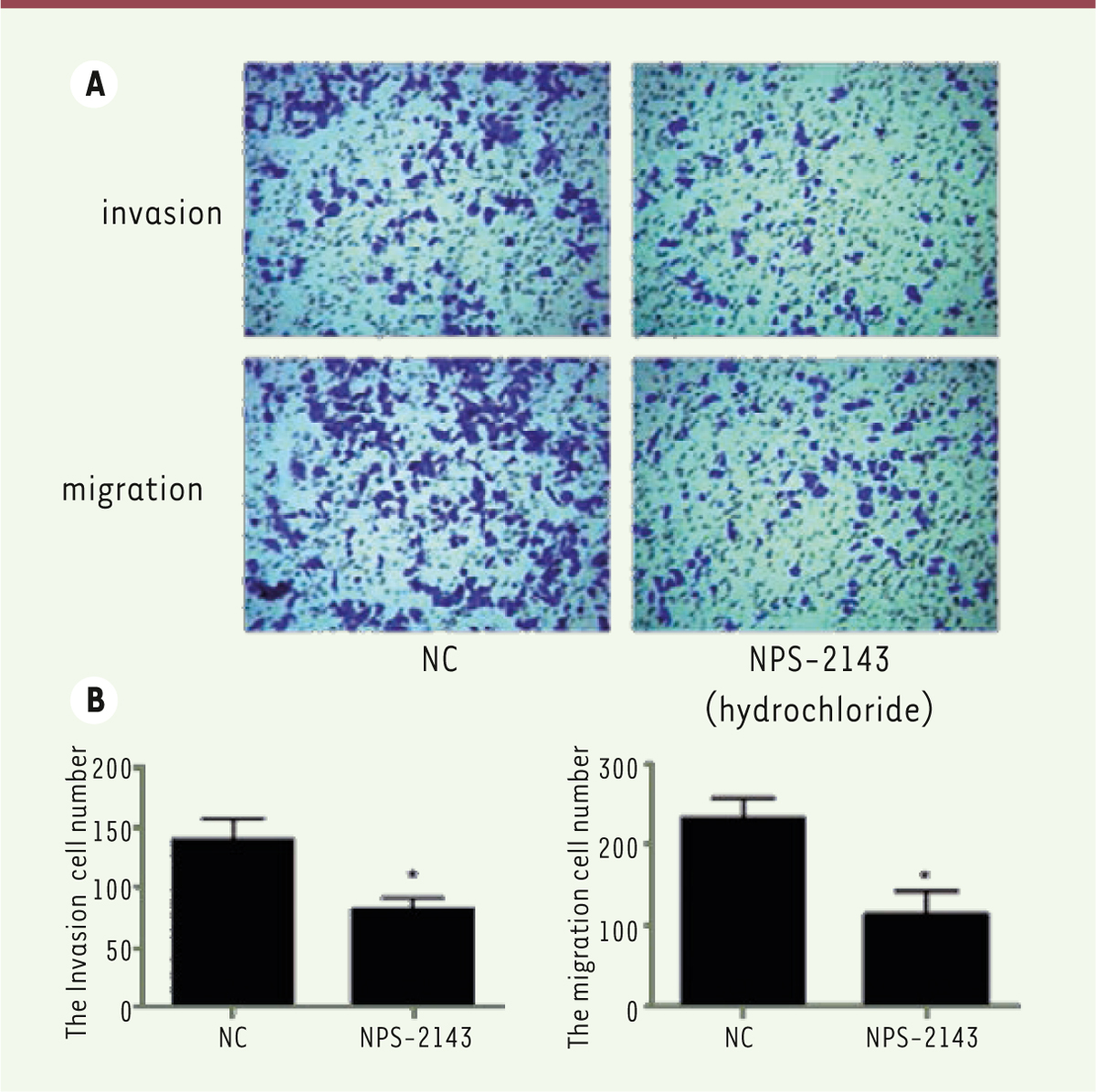
NPS-2143 treatment blocks the migration and invasion of M14 cells Trans-well assays make it possible to analyze the cancer cell migration and invasion in vitro. This assay is an in vitro system that enable to evaluate the migration and invasion capacity of malignant and normal cells. Thus, we investigated the effect of NPS-2143 treatment on M14 cell migration and invasion in vitro. Strikingly, the number of crystal violet stained cells in NPS-2143 treated wells was much lower than in the control groups ( Figure 4A). Also, the numbers of migrating and invasive cells were significantly decreased in NPS-2143-treated wells ( Figure 4B).
 | Figure 4.
NPS-2143 treatment inhibits migration and invasion of M14 cells. Transwell assays show that the migration of NPS-2143-treated M14 cells is significantly inhibited (*
P< 0.05). The invasion ability was also inhibited (*
P< 0.05). |
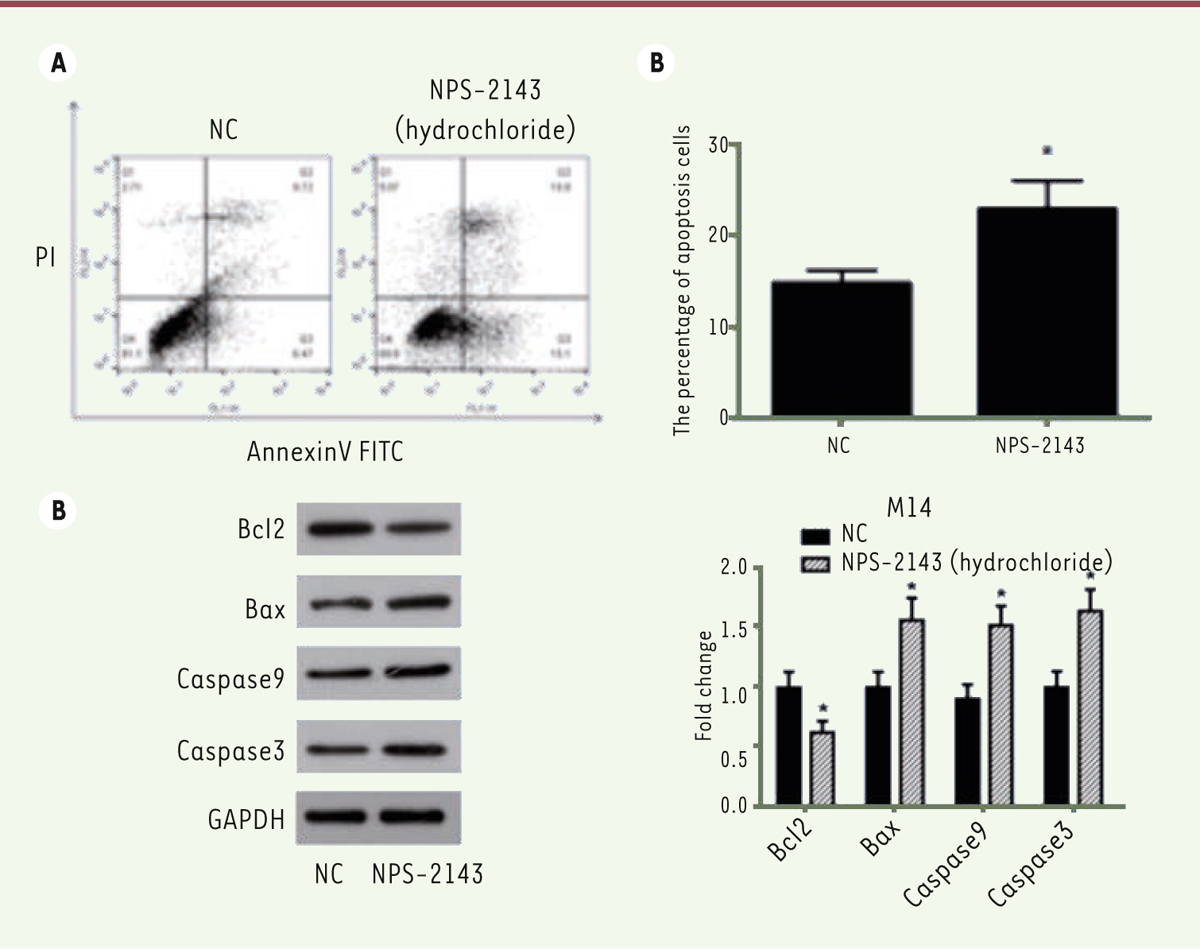
NPS-2143 induces apoptosis of M14 cells The apoptosis of M14 cells was analyzed by flow cytometry. Cells were treated with NPS-2143 for 24 hours, followed by a further incubation in serum free medium for another 24 hours to induce apoptosis. A double staining by Annexin V and PI was then performed. Our results showed that the treatment of NPS-2143 significantly increased the apoptosis rate of M14 cells compared with control cells ( Figure 5A and B).
 | Figure 5.
NP-2143 treatment induces apoptosis of M14 cells and regulates the expression of apoptosis‑related proteins. A. Flow cytometry staining reveals a significant shift in Annexin V-FITC-positive cells after NPS-2143 treatment of M14 cells. B. The percentage of apoptotic cells is shown (*
P < 0.05). C. M14 cell lysates were separated by SDS-PAGE and analyzed by western blots with the indicated antibodies. GAPDH was used as a loading control. D. The values of the band intensity represent the densitometric estimation of each band normalized to GAPDH (*
P < 0.05). |
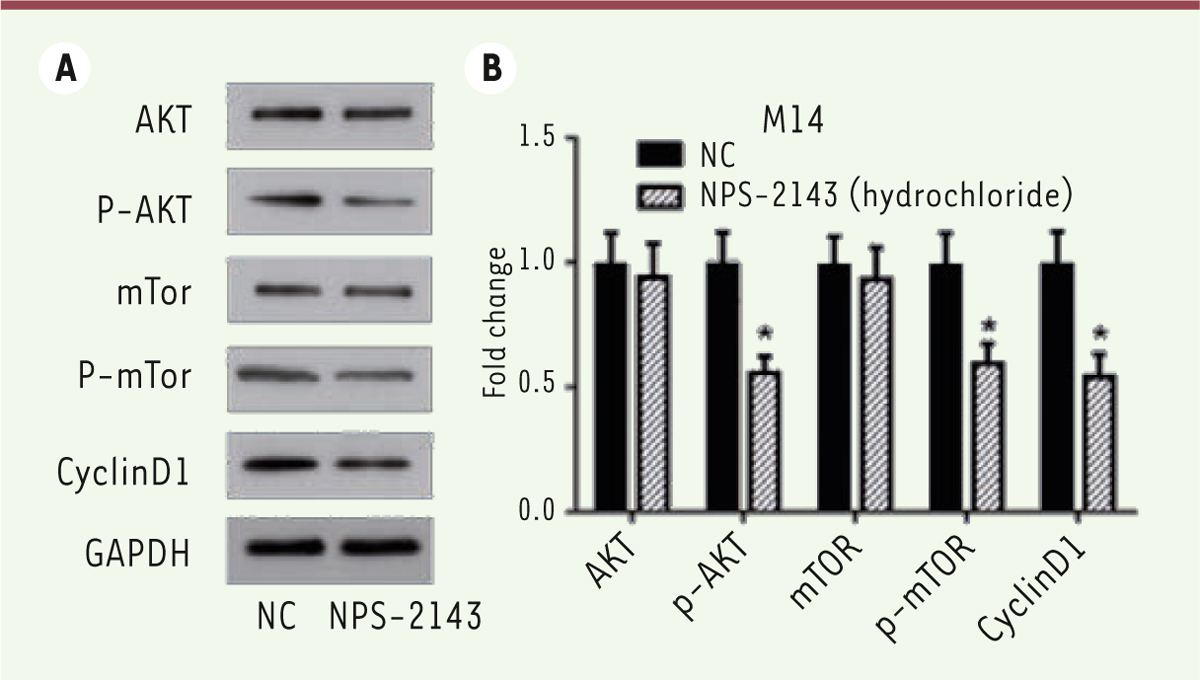
We then analyzed the expression of apoptosis‑related proteins by Western blots. Previous studies have shown that the expression of anti‑apoptotic Bcl‑2, pro‑apoptotic Bax, active caspase-3 and caspase-3 are critical factors for initiating apoptosis via mitochondria [19- 21], we examined the expression of Bcl‑2, Bax, active caspase-3 and caspase-9 in NPS-2143-treated and control M14 cells by Western blots. NPS-2143 treatment decreased the expression of Bcl‑2 while simultaneously increasing the expression of Bax, of active caspase-3 and caspase-9 ( Figure 5C and D). These results indicate that NPS-2143 increases the Bax/Bcl‑2 ratio in melanoma cancer cells, suggesting that NPS-2143 can inhibit melanoma cancer cells by triggering the mitochondrial apoptotic pathway. NPS-2143 treatment inhibits PI3K signaling pathway in M14 cells To study the molecular mechanism underlying the inhibitory activity of NPS-2143 against melanoma cancer cells, we analyzed the effect of NPS-2143 treatment on PI3K pathway in M14 cells. The PI3K signaling pathway plays important roles in many cellular processes such as cell growth, survival and promotion of angiogenesis. It is also a very important signaling pathway in cancer. The key proteins in this pathway such as Akt and mTOR are strongly involved in the proliferation of tumor cells. Once activated, PI3K phosphorylates PIP2, leading to the accumulation of PIP3 [ 22]. This lipid second messenger recruits PDK1 and Akt to the cell membrane, where Akt is phosphorylated by PDK1 [ 23]. Phosphorylated Akt regulates cellular processes through the phosphorylation of a number of substrates, including BclxL/Bcl-2-associated death promoter (BAD) [ 24]. Another Akt substrate, mTOR, has a major role in tumorigenesis [ 25]. When activated, mTOR increases mRNA translation by phosphorylating the downstream molecule P70-S6K [ 26]. Of note, P-70 is closely related to cell proliferation [ 27]. The western-blot results showed that, in NPS-2143-treated cells, there was no change in the expression of Akt and mTOR, while the phosphorylated form p-Akt and p-mTOR were significantly decreased ( Figure 6A and B). The expression of cyclin D1 was also found to be decreased ( Figure 6A and B). These data suggested that the treatment of NPS-2143 inhibits the PI3K pathway, with a marked decrease in the phosphorylated forms of Akt and mTOR.
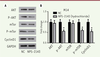 | Figure 6.
NPS-2143 treatment suppresses the activation of the PI3K signaling pathway in M14 cells. A. M14 cell lysates were separated by SDS-PAGE and analyzed by western blots with the indicated antibodies. GAPDH was used as a loading control. B. The values of the band intensity represent the densitometric estimation of each band normalized to GAPDH (*
P < 0.05) |
|
NPS-2143 is the first recognized Ca2+ receptor antagonists (calcilytics) which is used for the treatment of osteoporosis [14]. In the present study, we examined the effects of NPS on M14 human melanoma cancer cells. Interestingly, NPS-2143 provoked a strong inhibition of cell proliferation by inducing the apoptosis of these melanoma cells. Melanoma, which is the most aggressive type of skin cancer, has become more common since the 1960s in areas which are mostly populated with white people, both men and women [4, 11]. In 2015, newly occurred melanoma cases reached 3.1 million and 59,800 deaths were recorded [4, 28, 29]. Traditional treatment of melanoma is typically surgery [10]. Most people are cured if no metastasis is present [10]. At later stages, once melanoma have spread, patients receive immunotherapy, biologic therapy, radiation therapy, or chemotherapy in order to improve survival [10, 12]. The five-year survival rates after treatment is 98% in the United States among those with localized disease, while it is only 17% among those with metastases [13]. In the present work, we studied the effect of NPS-2143 on melanoma cancer M14 cells. NPS-2143 has been used for the treatment of osteoporosis [14] while there is no report on its effect on tumors. Herein, we have investigated the effect of NPS-2143 on melanoma cancer cell proliferation, migration and invasion by using M14 cells as a model and we have attempted to elucidate the underlying mechanisms. NPS-2143 treatment significantly inhibited the M14 proliferation, decreased migration and invasion by inducing apoptosis of these cells. Also, it affected the expression of apoptosis‑related proteins. Our results suggest that the effect of NPS-2143 on tumor cells involves the blockade of the PI3K signaling pathway. Bcl-2 associated X (BAX), a member of the Bcl-2 family, is a pro-apoptotic protein. Overexpression of Bax triggers the release of mitochondrial proteins that cleave and thereby activate caspase-3 and caspase-9, resulting in apoptosis [30]. In this study, we showed that treatment of NPS-2143 increases the Bax/Bcl‑2 ratio in M14 cells and that the expression of active caspase-3 is significantly increased. These data suggest that NPS-2143 triggers a mitochondria-related apoptosis. The PI3K/Akt/mTOR pathway is implicated in numerous cellular processes ranging from cell growth and survival to the promotion of angiogenesis [22, 31]. The serine/threonine kinase Akt inhibits apoptosis and mediates cell survival by activating phosphatidylinositol 3-kinase [32]. Another kinase, mTOR, is expressed in most mammalian cells [33], inhibiting autophagy and acting as a cellular sensor to nutrients and growth factors, as well as being an important effecter pathway of PI3K signaling [34]. β-catenin and stat3 signaling proteins are a family of proteins that play important roles in the development and maintenance of many tissues. We found that the NPS-2143 treatment decreases the phosphorylated form p-Akt and mTOR. Since the PI3K activation inhibits GSK3β, the increased expression of GSK3β and decreased expression of P70 further confirm this conclusion. Overall, in this study, we first reported that NPS-2143 could inhibit the proliferation and migration of the M14 melanoma cancer cells by inducing apoptosis via the inhibition of the activation of PI3K signaling pathway. Our findings suggest that NPS-2143 is a putative potent melanoma cancer inhibitor. Our results make this drug an exciting candidate for further experiments aimed at deciphering its role in the control of melanoma growth. |
The authors declare no competing or financial interests.
|
This study was supported by the China Postdoctoral Science Foundation (CPSF) (No.2014M550370, 2015T80740) and the Shandong Provincial Natural Science Foundation, China: No. ZR2017MH074).
|
1.
Epstein JI. Prostate cancer grading: a decade after the 2005 modified system . Modern Pathology An Official Journal of the United States & Canadian Academy of Pathology Inc. , 2018;;31::S47..
2.
Diao
K
Bian
SX
Routman
DM
et al. Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects . Journal of Neurosurgery. 2018; ; 1 : 3.
Rundle
P.
Photodynamic Therapy for Eye Cancer. Biomedicines. 2017; ; 5 : :69.. 4.
Epstein
JI
Prostate cancer grading: a decade after the 2005 modified system . Mod Pathol. 2018; ; 31 : :S47.–S63. 5.
Siegel
DA
King
J
Tai
E
et al. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009 . Pediatrics. 2014; ; 134 : :e945.–e955. 6.
Lee
JA
Current evidence about the causes of malignant melanoma . Prog Clin Cancer. 1975; ; 6 : :151.–161. 7.
Yu
GP
Hu
DN
McCormick
SA
Latitude and incidence of ocular melanoma . Photochem Photobiol. 2006; ; 82 : :1621.–1626. 8.
Kanavy
HE
Gerstenblith
MR
Ultraviolet radiation and melanoma . Semin Cutan Med Surg. 2011; ; 30 : :222.–228. 9.
Hu
S
Ma
F
Collado-Mesa
F
Kirsner
RS
UV radiation, latitude, and melanoma in US Hispanics and blacks . Arch Dermatol. 2004; ; 140 : :819.–824. 10.
Melanoma Treatment. (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries . Bethesda (MD): , 2002.
11.
Azoury SC, Lange JR. Epidemiology, risk factors, prevention, and early detection of melanoma . Surg Clin North Am. 2014;;94::945.–62, vii.
12.
Syn
NL
Teng
MWL
Mok
TSK
Soo
RA
De-novo and acquired resistance to immune checkpoint targeting . Lancet Oncol. 2017; ; 18 : :e731.–ee41. 13.
Fiddler
IJ
Melanoma Metastasis . Cancer Control. 1995; ; 2 : :398.–404. 14.
Nemeth
EF
Delmar
EG
Heaton
WL
et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone . J Pharmacol Exp Ther. 2001; ; 299 : :323.–331. 15.
Marquis
RW
Lago
AM
Callahan
JF
et al. Antagonists of the calcium receptor I. Amino alcohol-based parathyroid hormone secretagogues . J Med Chem. 2009; ; 52 : :3982.–3993. 16.
Kiefer
L
Leiris
S
Dodd
RH
Novel calcium sensing receptor ligands: a patent survey . Expert Opin Ther Pat. 2011; ; 21 : :681.–698. 17.
Breitwieser
GE
Pharmacoperones and the calcium sensing receptor: exogenous and endogenous regulators . Pharmacol Res. 2014; ; 83 : :30.–37. 18.
Gowen
M
Stroup
GB
Dodds
RA
et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats . J Clin Invest. 2000; ; 105 : :1595.–1604. 19.
Gross
A
McDonnell
JM
Korsmeyer
SJ
BCL-2 family members and the mitochondria in apoptosis . Genes Dev. 1999; ; 13 : :1899.–1911. 20.
Martinou
JC
Youle
RJ
Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics . Dev Cell. 2011; ; 21 : :92.–101. 21.
Norata
GD
Tonti
L
Roma
P
Catapano
AL
Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: possible role of oxidised LDL . Nutr Metab Cardiovasc Dis. 2002; ; 12 : :297.–305. 22.
Rameh
LE
Cantley
LC
The role of phosphoinositide 3-kinase lipid products in cell function . J Biol Chem. 1999; ; 274 : :8347.–8350. 23.
Downward
J.
Mechanisms and consequences of activation of protein kinase B/Akt . Curr Opin Cell Biol. 1998; ; 10 : :262.–267. 24.
Shaw
RJ
Cantley
LC
Ras, PI(3)K and mTOR signalling controls tumour cell growth . Nature. 2006; ; 441 : :424.–430. 25.
Raught
B
Gingras
AC
Sonenberg
N
The target of rapamycin (TOR) proteins . Proc Natl Acad Sci USA. 2001; ; 98 : :7037.–7044. 26.
Jefferies
HB
Fumagalli
S
Dennis
PB
et al. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k . EMBO J. 1997; ; 16 : :3693.–3704. 27.
Li
DM
Song
WW
Wang
Y
Fu
JL
The investigations on the relationship between the birth weight of fetus and the P70 S6K as well as mechanism insulin regulate the birth weight of fetus . Zhonghua Yi Xue Za Zhi. 2009; ; 89 : :2924.–2927. 28.
Disease
GBD
Injury
I
Prevalence
C
Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet. 2016; ; 388 : :1545.–1602. 29.
Mortality
GBD
Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet. 2016; ; 388 : :1459.–1544. 30.
Eskes
R
Antonsson
B
Osen-Sand
A
et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions . J Cell Biol. 1998; ; 143 : :217.–224. 31.
Wang
Z
Wang
N
Han
S
et al. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway . PLoS One. 2013; ; 8 : :e68566.. 32.
Stal
O
Perez-Tenorio
G
Akerberg
L
et al. Akt kinases in breast cancer and the results of adjuvant therapy . Breast Cancer Res. 2003; ; 5 : :R37.–R44. 33.
Brown
EJ
Albers
MW
Shin
TB
et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex . Nature. 1994; ; 369 : :756.–758. 34.
Ersahin
T
Tuncbag
N
Cetin-Atalay
R
The PI3K/AKT/mTOR interactive pathway . Mol Biosyst. 2015; ; 11 : :1946.–1954. |