| |
| Med Sci (Paris). 34: 4–7. doi: 10.1051/medsci/201834f101.Liuwei Dihuang exhibits antidiabetic effects through inhibiting α-amylase and α-glucosidase Huafu Wang,1# Huang Gang,2# Shuxin Zhou,3 Lixian Liu,1 Ting Ding,1 Zhihong Gui,4* and Weihua Chu2* 1Department of pharmacy, Lishui People’s Hospital, the Sixth Affiliated Hospital of Wenzhou Medical University, Lishui323000, China 2Department of Traditional Chinese Medicine, Lishui People’s Hospital, the Sixth Affiliated Hospital of Wenzhou Medical University, Lishui323000, China 3School of Life Science and Technology, China Pharmaceutical University, Nanjing210009, P.R. China 4Department of Nephrology, Lishui People’s Hospital, the Sixth Affiliated Hospital of Wenzhou Medical University, Lishui323000, China |
Objective: Liuwei Dihuang (LWDH) is a famous traditional herbal medicine formula in China that may regulate the balance of kidney yin yang and has been used to restore functional insufficiency of the kidney for a long time in China. Methods: In this study, the water extract of LWDH was tested for its α-Amylase and α-Glucosidase inhibitory activities, and its anti-diabetic property in streptozotocin (STZ)-induced diabetic mice was also analyzed. Results: LWDH extract inhibited α-Glucosidase and α-Amylase activities in a dose- dependent manner. Treatment of streptozotocin-induced diabetic mice with LWDH extract decreased camp, fasting blood glucose, TC, TG, LDL-c, HbA1C, Urine volume levels and Urine sugar, increased HDL-c level when compared to STZ induced diabetic mice. Conclusion: This study demonstrates that extract of LWDH can inhibit α-amylase and α-glucosidase activities and shows anti-diabetic effect in a mice preclinical model. Keywords: Liuwei Dihuang, antidiabetic effects, α-amylase, α-glucosidase |
Diabetes mellitus (DM) is not a single disease, but a heterogeneous group of metabolic diseases characterized by altered lipid, carbohydrate and protein metabolism which causes hyperglycemia [1,2]. Many studies have demonstrated that Chinese traditional herbs possess a wide range of pharmacological activities in retarding progressive chronic kidney diseases [3]. In China, a lot of different herbs are used for treating DM in order to decrease blood sugar level, including yin nourishing herbs and heat-clearing herbs. Liuwei Dihuang (LWDH) is a famous traditional Chinese herbal medicine formula that may nourish the balance of kidney yin yang that has been used to restore functional insufficiency of the kidney, liver, spleen and other “Kidney-Yin” deficiency syndrome for a long period of time in China [4]. It is comprised of 6 Chinese herbs, including Radix Rehmanniae (Dihuang; prepared root of Rehmannia glutiosa), Rhizoma Dioscoreae (Shanyao; rhizome of Dioscorea opposita), Fructus Corni (Shanzhuyu; fruit of Cornus officinalis), Cortex Moutan Radicis (Mudanpi; root bark of Paeonia suffruticosa), Rhizoma Alismatis (Zexie; rhizome of Alisma plantago-aquatica) and Poria (Fuling; scleorotia of Poria cocos) [5]. In clinical practices, Liuwei Dihuang was commonly used for adjuvant treatment of patients with Type 2 diabetes. However, the mechanism of the observed antidiabetic effects was not absolutely clear. The aim of the present study was to evaluate the α-glucosidase and α-amylase inhibitory activities of the Chinese herbal formulation, LWDH, and its anti-diabetic activity in streptozotocin (STZ)-induced diabetic mice |
Preparation of Liuwei Dihuang extract Six different constituent medicinal plants of Liuwei Dihuang are shown in Table 1. The traditional herbal medicines were purchased from Nanjing Tongrentang Pharmaceutical Co., Ltd. The procedure of preparing LWDH extracts was as follow: 1) Seventy-five (g) purchased Chinese herbal medicine were added in 1.5 L pure water and boiled for 120 minutes. 2) After centrifugation, the supernatant was removed, 1 L boiling water was then added and incubated for 2 h (repeated 3 times). 3) The supernatant was then carefully mixed, filtered, and then properly concentrated in a rotary evaporator. 4) The extracts were condensed to 2g/mL in sterile distilled water and kept at -20°C until use.
Table 1
| Plant name |
Proportions (g) |
| Radix Rehmanniae |
24 |
|
| Rhizoma Dioscoreae |
12 |
|
| Fructus Corni |
12 |
|
| Cortex Moutan Radicis |
9 |
|
| Rhizoma Alismatis |
9 |
|
| Poria cocos
|
9 |
Plant origin and relative proportions in LWDH extracts. |
α-Amylase and α-Glucosidase inhibitory properties of water extract of LWDH The amylase inhibition assay was performed as described by Prabhakar [ 6]. The α-glycosidase inhibitory activity of the extracts was determined according to the method described by Apostolidis and Lee with slight modifications [ 7]. A mixture of 630 μL of sample, including 40 μL of extracts, 20 μL of 3 mM glutathione diluent with ddH 2O, 50 μL of 5 mM p-nitrophenyl--galactoside (PNPG) solution, 500 μL of 0.1 M phosphate buffer (pH 6.9), and 20 μL of α-glycosidase solution (1 U/mL) was added to each centrifuge tube and incubated at 37°C for 20 min. Next, 40 μL of reaction mixtures were placed into 96-well plates, and 160 μL of 0.1 M sodium carbonate solution were added to end the reaction. Absorbance was recorded at 400 nm using a microplate reader. The α-glycosidase inhibitory activity was expressed as the inhibition percentage and was calculated as follows: inhibition (%) = (1 – Δ Asam/Δ Acon) × 100%. Δ was defined as absorbance of the sample or of the control. Animals Eight-weeks-old male C57BL/6J mice were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). The mice were housed in an animal room at constant temperature (25±2°C), relative humidity (55%±5%) and allowed free access to water and standard laboratory chows under a 12-h light–12-h dark cycle in the animal facility at the China Pharmaceutical University. All experimental procedures involving animals were approved by the ethical animal committee of the China Pharmaceutical University, China. Diabetes mice modeling After 2 weeks of acclimatization, all the mice were considered to be healthy. Diabetes was induced in female mice with streptozotocin (STZ, Sigma-Aldrich, St. Louis, MO, USA). Citrate buffer (0.1 M, pH 6.5) was used to dissolve STZ that was injected intraperitonally (40 mg/kg). Mice in control group were given the same volume of citrate buffer. The blood glucose was measured seven days after induction of diabetes. DM was proved when glucose concentration exceeded 200 mg / dL. Grouping and treatment The mouse were divided into three groups (n=8 per group) randomly as follows: normal group (NC): mice without diabetes; Diabetic control group (DC): mice with diabetes withouttreatment; and the Liuwei dihuang-treated group (LWDH): mice with diabetes and treated with LWDH, based on the previously reported dosage of 2.4 g/kg/d in mice [ 8]. Observation index Body weights were measured and biochemical variables such as fasting blood glucose (FBG), urine sugar, urine volume, serum cyclic adenosine monophosphate (cAMP), glycated hemoglobin (HbA1C), serum total cholesterol (TC), serum (HDL-c), low density lipoprotein cholesterol (LDL-c), serum triglyceride (TG) were determined by a commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the protocols and using an automatic biochemical Abbott-Aeroset autoanalyzer (Chicago, IL, USA). Statistical analysis All the data were analyzed using the SPSS18.0 software ( http://www-01.ibm.com/software/analytics/spss/). The measurement data such as weight and glucose level were evaluated by calculating the mean value ± standard deviation (  ). Data of different groups compared from multiple time were analyzed using the ANOVA test. Results with P<0.05 were considered as statistically significant. |
α-amylase and α-glucosidase inhibition by LWDH extract
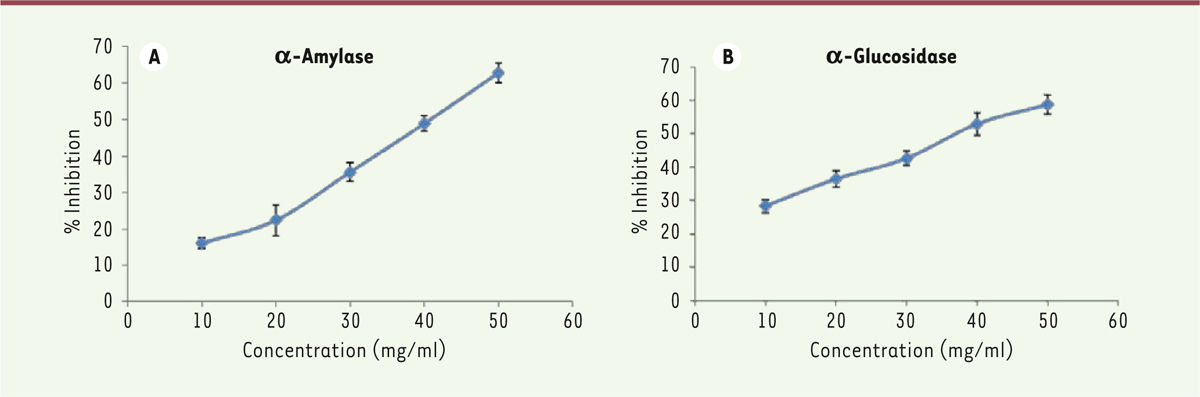
Inhibition of α-Amylase and α-Glucosidase can significantly reduce the post-prandial glucose level. To explore the underlying mechanism of blood glucose-lowering effects of the extracts, the inhibitory effects of LWDD on α-Amylase and α-Glucosidase activity were assayed in vitro. α-Amylase inhibitory activities of LWDH are shown in Figure 1a, LWDH induced a dose-dependent inhibitory effect on α-amylase. The extracts exerted an inhibitory activity in varying proportions (16.1-62.8% at the concentration of 10-50 mg/mL). The LWDH extracts induced 50% enzyme inhibition (IC50) at 40.79 mg/mL. The inhibitory activity of water extract of LWDH on a-Glucosidase was evaluated at 5 different doses between 10–50 mg/mL; LWDH exerted a concentration-dependent suppression on α-glycosidase activity: 28.3% to 58.7% at the concentration of 10 mg/mL to 50 mg/mL (Figure 1B), the calculated IC50 value was 38.05 mg/mL.  | Figure 1 Inhibitory activities of LWDH extracts against α-amylase (A) and α-glycosidase (B). The experiment was performed in triplicates. |
Changes in diabetic parameters The body weight changes of mice in different groups are shown in Table 2. The body weights of all the mice gradually increased over the experimental period, with body weight in NC group mice increasing slowly during three weeks. Of note, the body weights in the DC group were significantly higher ( P<0.05) than those in the NC and LWDH groups.
Table 2
| Group |
0-W |
1W |
2-W |
3-W |
| NC |
25.15±3.42 |
26.45±3.42 |
26.92±2.67 |
27.28±2.46* |
|
| DC (STZ) |
25.31±2.47 |
27.67±3.45 |
30.24±3.38 |
32.43±2.92 |
|
| LWDH |
25.03±2.78 |
25.41±5.06 |
26.53±4,87 |
27.10±3.52* |
Effect of LWDH on body weight in STZ diabetic mice (n=8). Data were analyzed by one-way ANOVA. Results are presented as mean values with their standard errors (n8). |
The effect of LWDH extracts on the FBG, cAMP, HbA1C, serum TC, serum HDL-c and LDL-c, serum TG, urine sugar and urine volume in all groups mice is shown in Table 3. After a 3 week treatment, diabetic control group and LWDH-treated group mice showed an increase in FBG, cAMP, HbA1C, serum TC, and LDL-c, serum TG, urine sugar levels and urine volume but a decrease in serum HDL-c level when compared to normal mice. However, LWDH-treated diabetic mice showed a significant decrease of FBG, cAMP, HbA1C, serum TC, LDL-c, serum TG, urine sugar and urine volume levels, but an increased serum HDL-c level when compared to diabetic mice.
Table 3
| Group |
FBG(mmoL/L) |
cAMP(mmoL/L) |
TC(mmoL/L) |
TG(mmoL/L) |
HDL C(mmoL/L) |
LDL C(mmoL/L) |
HbA1C(%) |
Urine sugar (mg/24 h/L) |
Urine volume (ml/24 h) |
| NC |
6.1±0.4 |
0.97±0.04 |
1.51±0.09 |
0.84±0.07 |
1.14±0.07 |
0.45±0.03 |
24±0.48 |
83.4±28.8 |
1.3±0.15 |
|
| DC (STZ) |
18.1±2.3a**
|
1.26±0.023a**
|
2.20±0.17a**
|
1.58±0.12a**
|
0.75±0.06a**
|
1.06±0.04a**
|
10.8±1.03a***
|
1000.0±12.6a***
|
4.16±0.97a***
|
|
| LWDH |
13.9±0.5b*
|
1.18±0.03b*
|
1.92±0.12b**
|
1.36±0.05b**
|
0.89±0.03b*
|
0.82±0.03b**
|
7.3±0.87b***
|
90.0±22.4b***
|
1.56±0.32b**
|
Effect of LWDH extract on various diabetic parameters in STZ-induced diabetic mice (n=8). All values represent mean ± SD |
|
Diabetic patients, in both fasting and post-prandial states, should be treated by maintaining glycemic control near-normal. Glucose generation from carbohydrates in the gut or its absorption from the intestine have been inhibited by using many natural resources [9]. The hydrolysis of α-1,4-glucosidic linkages in starch, glycogen and various oligosaccharides can be catalyzed by α-amylase, and disaccharides are further decomposed by α-glucosidase into simpler sugars, allowing easy intestinal absorption. Diabetes can be effectively controlled due to the inhibition of their activities in the digestive tract of human through decreasing the absorption of starch-derived glucose [10]. In China and many developing countries, traditional medicine, especially traditional Chinese herbal, may be the only affordable sources for health care. Herbal drugs are widely prescribed owing to satisfactory therapeutic effects, mild side effects and low costs. Based on this, researchers have endeavored to develop novel drugs for diabetes by including eligible traditional medicines from various cultures. In the present study, LWDH extracts dose-dependently inhibited the activities of α-amylase and α-glucosidase. Similarly, for mice whose diabetes was induced by streptozotocin, LWDH extracts reduced the levels of fasting blood glucose, TC, TG, LDL-c, cAMP, HbA1 as well as urine volume, but raised that of HDL-c. In 2008, Bhandari et al. reported that the herb Bergenia ciliate had the same effects and may be used to treat diabetes, obesity, cancers, and other chronic diseases [11]. Although the enzyme-inhibitory activities of LWDH were detected in vitro and in mice in this study, these results should be confirmed in other preclinical models before clinical use in Humans. Of note, the inhibitory activities of Chinese herbs against α-glucosidase and α-amylase have been determined by different in vitro and in vivo models [9, 11, 12] arguing in favor of the presence of anti-DM molecules in the Chinese Herbs extracts that need to be further characterized. In summary, for the first time, we provide valuable evidence for the LWDH use in the treatment of diabetes through its inhibitory effects on α-glucosidase and α-amylase. This study is helpful to elucidate the pharmacological mechanisms of LWDH and associated symptoms. |
The authors declare that there is no conflict of interests regarding the publication of this paper. |
This study was supported in part by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and High-Level Talents Training Project of Lishui, Zhejiang Province (2014RC17).
|
-
Tibaldi JM
Intensifying insulin therapy in type 2 diabetes mellitus: dosing options for insulin analogue premixes.
Clinical therapeutics.
2011;
33
(11)
:1630.
42
-
Zhu K
Guo S
Liang X
Diabetes mellitus treated by traditional Chinese medicine.
Journal of the American College of Traditional Chinese Medicine.
1983;
1
(1)
:24.
30
-
Peng A
Gu Y
Lin S
Herbal treatment for renal diseases.
Ann Acad Med Singapore.
2005;
34
(1)
:44.
51
-
Ye J
Zhang X
Dai W
Yan S
Huang H
Liang X
Chemical fingerprinting of Liuwei Dihuang Pill and simultaneous determination of its major bioactive constituents by HPLC coupled with multiple detections of DAD, ELSD and ESI-MS.
Journal of pharmaceutical and biomedical analysis
.
2009;
49
(3)
:638.
45
-
Xie B
Gong T
Tang M
Mi D
Zhang X
Liu J
An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers.
Journal of pharmaceutical and biomedical analysis
.
2008;
48
(4)
:1261.
6
-
Prabhakar VK
Jaidka A
Singh R
In vitro study on α-amylase inhibitory activity and phytochemical screening of few Indian medicinal plant having anti-diabetic properties.
Citeseer.
2013;
34
(2)
:44.
51
-
Apostolidis E
Lee C
In Vitro potential of ascophyllum nodosum phenolic antioxidant mediated α-glucosidase and α-amylase inhibition.
Journal of food science
.
2010;
75
(3)
:H97.
H102
-
Xue Y
Luo R
Zhu B
Zhang Y
Pan Y
Li C
Effects of liuwei dihuang pills on expressions of apoptosis-related genes bcl-2 and Bax in pancreas of OLETF rats.
Journal of Chinese integrative medicine.
2005;
3
(6)
:455.
8
-
Matsui T
Tanaka T
Tamura S
Toshima A
Tamaya K
Miyata Y
α-Glucosidase inhibitory profile of catechins and theaflavins.
Journal of Agricultural and Food Chemistry.
2007;
55
(1)
:99.
105
-
Hara Y
Honda M
The inhibition of α-amylase by tea polyphenols.
Agricultural and Biological Chemistry.
1990;
54
(8)
:1939.
45
-
Braca A
Politi M
Sanogo R
Sanou H
Morelli I
Pizza C
Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves.
Journal of agricultural and food chemistry.
2003;
51
(23)
:6689.
95
-
He Q
Lv Y
Yao K
Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase.
Food Chemistry.
2007;
101
(3)
:1178.
82
|


 ). Data of different groups compared from multiple time were analyzed using the ANOVA test. Results with P<0.05 were considered as statistically significant.
). Data of different groups compared from multiple time were analyzed using the ANOVA test. Results with P<0.05 were considered as statistically significant.